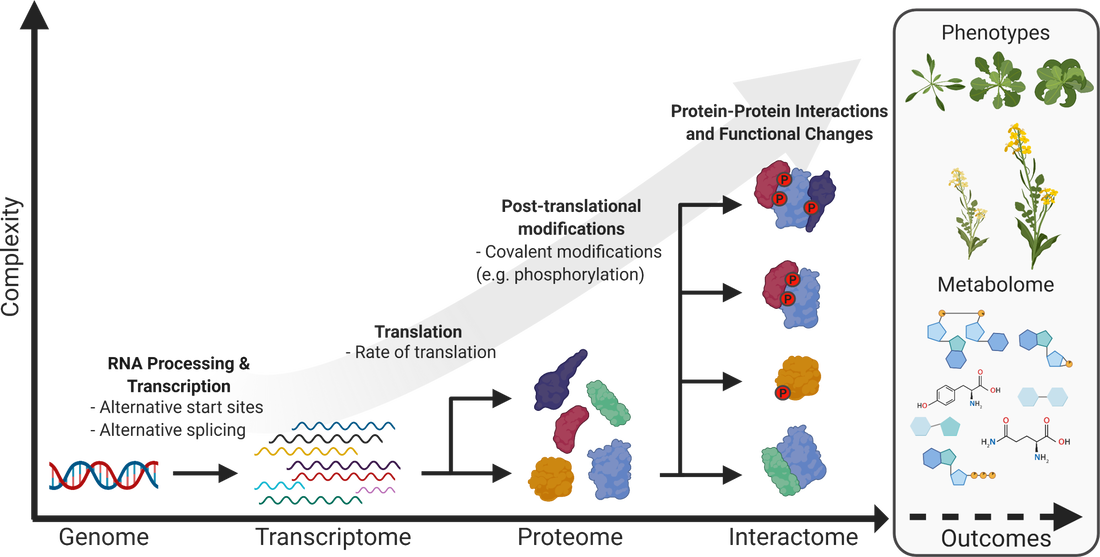
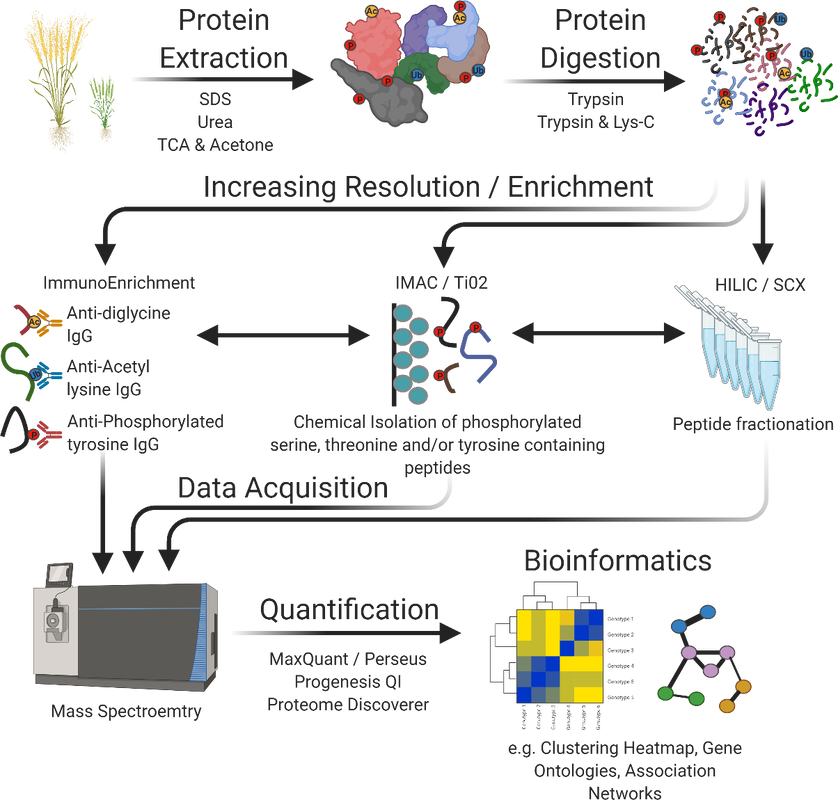
Our lab utilizes functional genomic technologies to characterize a wide range organisms. In particular, we are focused on resolving the signaling events that control plant growth and development under changing light and stress conditions.
|
|
To do this, we employ quantitative proteomics to examine the model plant Arabidopsis thaliana and a multitude of Agriculturally relevant crops from a systems perspective. Targets of interest are further investigated using a combination of molecular and cellular biology, biochemistry as well as targeted proteomics.